Multi-site, prospective studies, and a patient registry, to commence in multiple countries with a total of 150 patients to be enrolled – titled the HEAL Registry Study
Salt Lake City, Utah and Playa Vista, CA – November 27th, 2017– VascuStim, an emerging leader in advanced bioelectric innovations and technologies for peripheral vascular diseases, today announced the launch of a clinical research program which will comprise multiple studies and an international patient registry.
VascuStim is a class II medical device produced on OEM private label basis by Mettler Electronics of Anaheim, California that has been 510(k) cleared by the US Food and Drug Administration (FDA) and is intended to improve blood circulation, accelerate healing and provide mild pain relief. The device is programmed with VascuStim’s patented and patent pending signals and sequences designed for optimally improving blood circulation and healing.
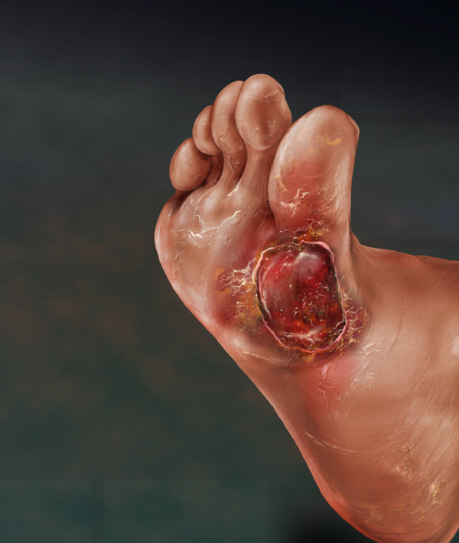
The international registry study will seek to gather data to better understand VascuStim’s utilization as a tool for accelerating the healing of diabetic foot and leg ulcers. The registry will also examine the potential capabilities of the device and specific bioelectric signals towards improving blood circulation so that wounds do not return and relieving the patients of associated wound related pain.
“Up until now, we’ve seen encouraging case studies showing individual patient results following treatment with bioelectric stimulation and amniotic fluid membranes” stated Dr. Leslie Miller, Chief Medical Officer for VascuStim “While those studies have yielded valuable information, the completion of this 150 plus patient prospective research program will provide wound care clinicians with important clinical data regarding how VascuStim and amniotic fluid membrane dressings may be utilized specifically for diabetic foot and leg ulcer care and management”
“We are very excited to be at the forefront of this important research initiative to further our understanding of the full therapeutic potential this unique product,” stated Howard Leonhardt, Executive Chairman and CEO of VascuStim.
The prospective international HEAL Registry will examine and assess the use of VascuStim’s bioelectric stimulation protocol in real world clinical settings. Study objectives seek to evaluate the effectiveness of VascuStim as a diabetic foot and leg ulcer wound management product as indicated by percent reduction in wound size, time to complete wound closure, and improvements in wound bed condition. The Heal Registry will also assess increases in healthy granulation tissue, reduction in biofilm, and readiness for advanced therapy applications such as amniotic fluid membrane dressing changes. Full leg blood flow circulation improvement will also be assessed. Patients will be followed for a for a minimum of 24 weeks.
The HEAL Registry will also collect patient reported pain and quality of life information before and after the use of VascuStim, as well as economic outcome information.
“VascuStim is committed to developing and manufacturing innovative products backed by sound clinical research and scientific data that serve our customers’ needs,” noted Brett Burton, Director of R&D. “We look forward to sharing the valuable findings of both the VascuStim clinical research program and the HEAL Registry with the entire diabetic foot and leg ulcer wound care community.”
About VascuStim: VascuStim (formerly MyoStim Peripheral) was founded in 2013 by Leonhardt Ventures and is currently incubating in the Leonhardt’s Launchpads innovation and startup accelerators in California and Utah. Howard Leonhardt the Founder and Lead Inventor has over 21 issued U.S. patents related to organ regeneration and recovery and dozens more patent claims pending. The Leonhardt team has helped lead dozens of electrical stimulation, stem cell and growth factor related studies for improving blood circulation and promoting organ regeneration and recovery since 1988. VascuStim was formed in 2013 to focus all this accumulated knowledge, experience, technology platform, building patent portfolio and all-star assembled team on treating peripheral vascular diseases. The Leonhardt team previously patented, developed and brought to market the leading endovascular aortic stent graft utilized by peripheral vascular physicians around the world today. Over 400,000 patients have been treated with Leonhardt cardiovascular related inventions to date.
About Leonhardt Ventures: Leonhardt Ventures (Leonhardt Vineyards LLC) was originally formed as H.J. Leonhardt & Co. in 1982. It is the commercialization arm for inventor Howard J. Leonhardt. Leonhardt has 21 issued U.S. patents focused on organ regeneration and recovery and has dozens more new patent claims pending with the U.S.P.T.O. https://patents.justia.com/inventor/howard-j-leonhardt Leonhardt is most well known for improved cardiovascular balloon catheters patented and developed in the 1980’s, stent grafts, percutaneous heart valves and biological pacemakers developed in the 1990’s and stem cell and bioelectric organ regeneration devices and therapies developed since 2000. See www.leonhardtventures.com for more information and our Annual Report.
About Mettler Electronics: Founded in 1957 by engineer and inventor, Hal Mettler, the firm is the world leader in portable ultrasound and electro-stimulation therapy equipment used in sports medicine, physical therapy, chiropractic and podiatric health care. Mettler’s customers include US Olympic Trainers, professional teams like the Los Angeles Angels of Anaheim and Dallas Cowboys, as well as countless health care institutions and individual practitioners.
For more information please visit www.vascustim.com.
CONTACT:
Asli Gozoren
Director of Investor Relations
Brian Hardy, Director of Marketing, brian@leonhardtventures.com
Howard J. Leonhardt, Executive Chairman & CEO, howard@leonhardtventures.com
VascuStim is incubating in California and Utah at these locations:
Leonhardt’s Launchpads by Cal-X Stars Business Accelerator, Inc., 12655 W Jefferson Blvd, Los Angeles, CA 90066 – www.leonhardtventures.com + www.calxstars.com
Leonhardt’s Launchpads Utah, Inc. 370 S, 300 E, Salt Lake City, UT 84111
Research Lab @ 2500 S State St. #224, Salt Lake City, UT 84115
Leonhardt Ventures LLC, 12760 Millennium Dr., Unit 213, Playa Vista, CA 90094