Restoring Blood Flow to Limbs. Saving Legs.
Specializes in bioelectric signaling
technologies that are redefining
regeneration, healing, and recovery.
Learn More
technologies that are redefining
regeneration, healing, and recovery.
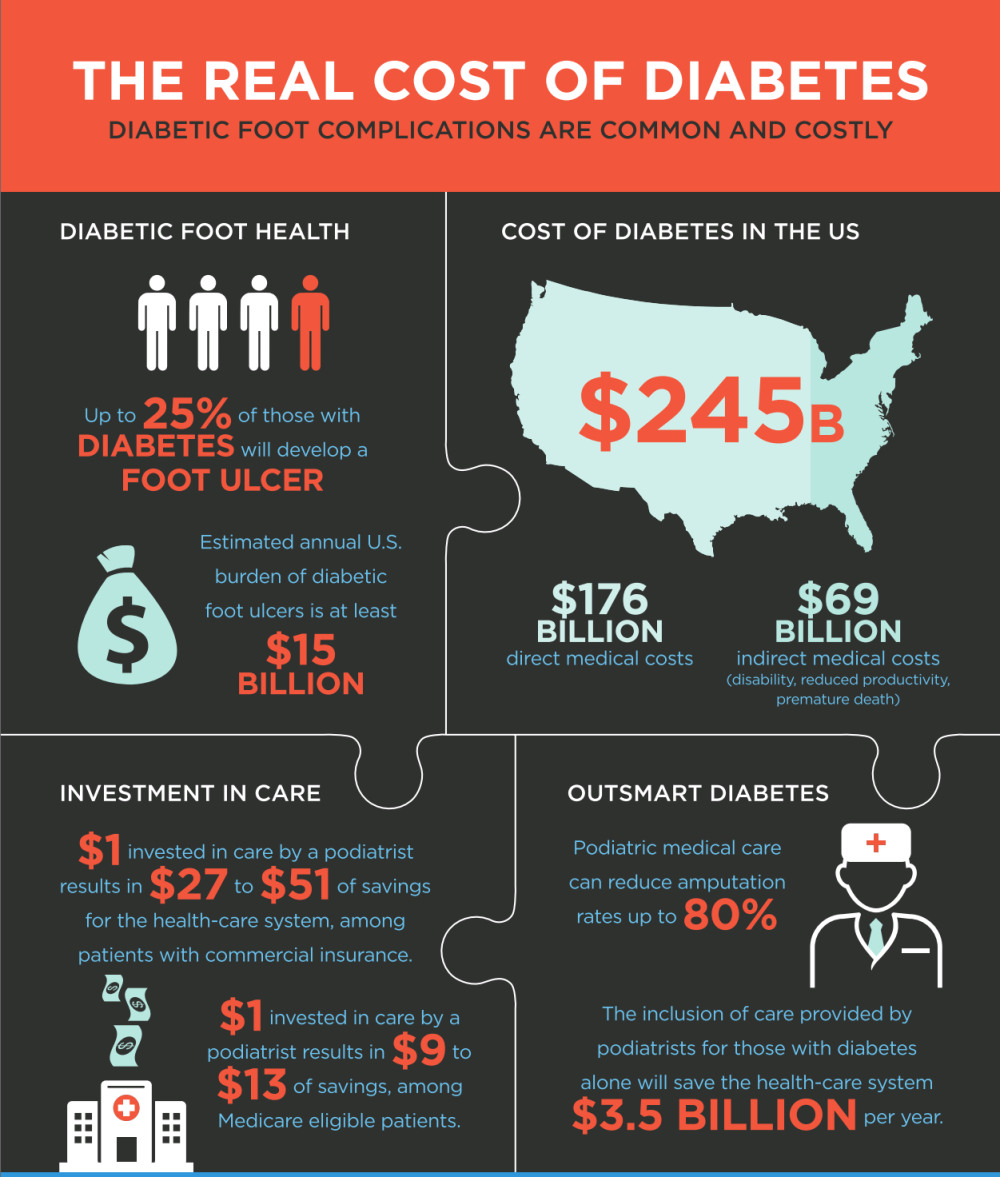
VascuStim is in the business of developing treatments utilizing the innovative advancements in bioelectric stimulation controlled protein expression, stem cell homing, proliferation and differentiation combined with stem cel based mixed compositions such as amniotic fluid which has over 240 known growth factors critical and important to healthy tissue and organ development and repair. We committed to gathering data in well controlled studies with patient safety always established first and foremost. Our stem cell based compositions are procured and handled by an FDA HCT/P certified lab. The company prides itself on its state-of-the-art technology and its ability to provide products of the highest standards in the industry. Our amniotic fluid and membranes are processed from donated human tissue from full term deliveries and are regulated as a human cell, tissue, or cellular or tissue-based product (HCT/P) under 21 CFR Part 1271 and Section 361 of the Public Health Service Act.
The impact of continuous electrical microcurrent on acute and hard-to-heal wounds: a systematic review
Your content goes here. Edit or remove this text inline or in the module Content settings. You can also style every aspect of this content in the module Design settings and even apply custom CSS to this text in the module Advanced settings.
Bioelectric + light therapy stimulation and amniotic fluid combination designed to treat:
1. Critical limb ischemia.
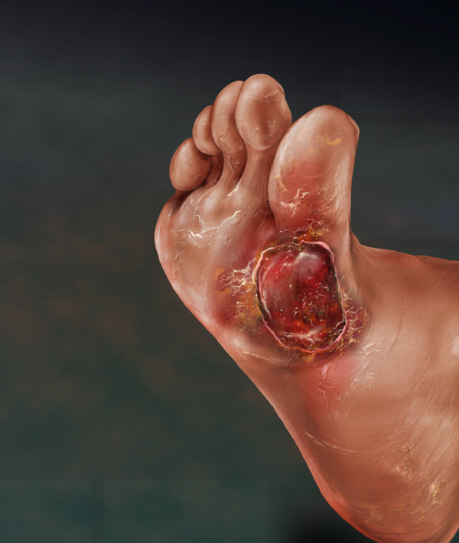
2. Diabetic foot and leg ulcers.
3. Diabetic neuropathy.
4. Limb salvage.
VascuStim’s mission is to harness the power of precise bioelectric signaling.
71% Electrical Stimulated Ulcers Healed only
39% in Placebo Control Group Healed
39% in Placebo Control Group Healed
1 Year Out Diabetic Foot Ulcers Treated with Best Standard Current Therapies
- 44.5% healed
- 17.4% AMPUTATED!
- 38.1% NOT HEALED!
5 years out data is much worse!
- Outcomes for people with a clinically infected diabetic foot ulcer are poorer than previously thought; in the first year after presentation with an infected ulcer, 15.1% of our participants had died and 17.4% underwent at least partial lower extremity amputation.
- Healing incidence within 1 year was 44.5% (95% CI 38.9 to 50.1). Three key factors served as the best independent predictors of healing: PEDIS (perfusion, extent, depth, infection, sensation) perfusion grade; the absence of multiple foot ulcers; and shorter ulcer duration.
Get ahold of us
CAUTION Disclaimer and Warning: Products described on this web site are in early stage development and are not yet proven safe or effective in statistically significant controlled clinical studies. Any statement or phrases implying efficacy or safety in any form are considered modified by “intended to” or “designed to”. Investigational use only in countries where investigation is permitted by law and proper filings have been made and appropriate regulatory clearances have been granted. Any use of the product(s) must be in an authorized clinical study with institutional review board (ethics committee) approval and proper patient consent procedures followed. For other countries product is only available for laboratory investigation by credentialed institutions and investigators with proper clearances with a research agreement in place with a study sponsor. NOT AVAILABLE FOR SALE.
CAUTION: Investigational device. Limited by Federal (or United States) law to investigational use.
